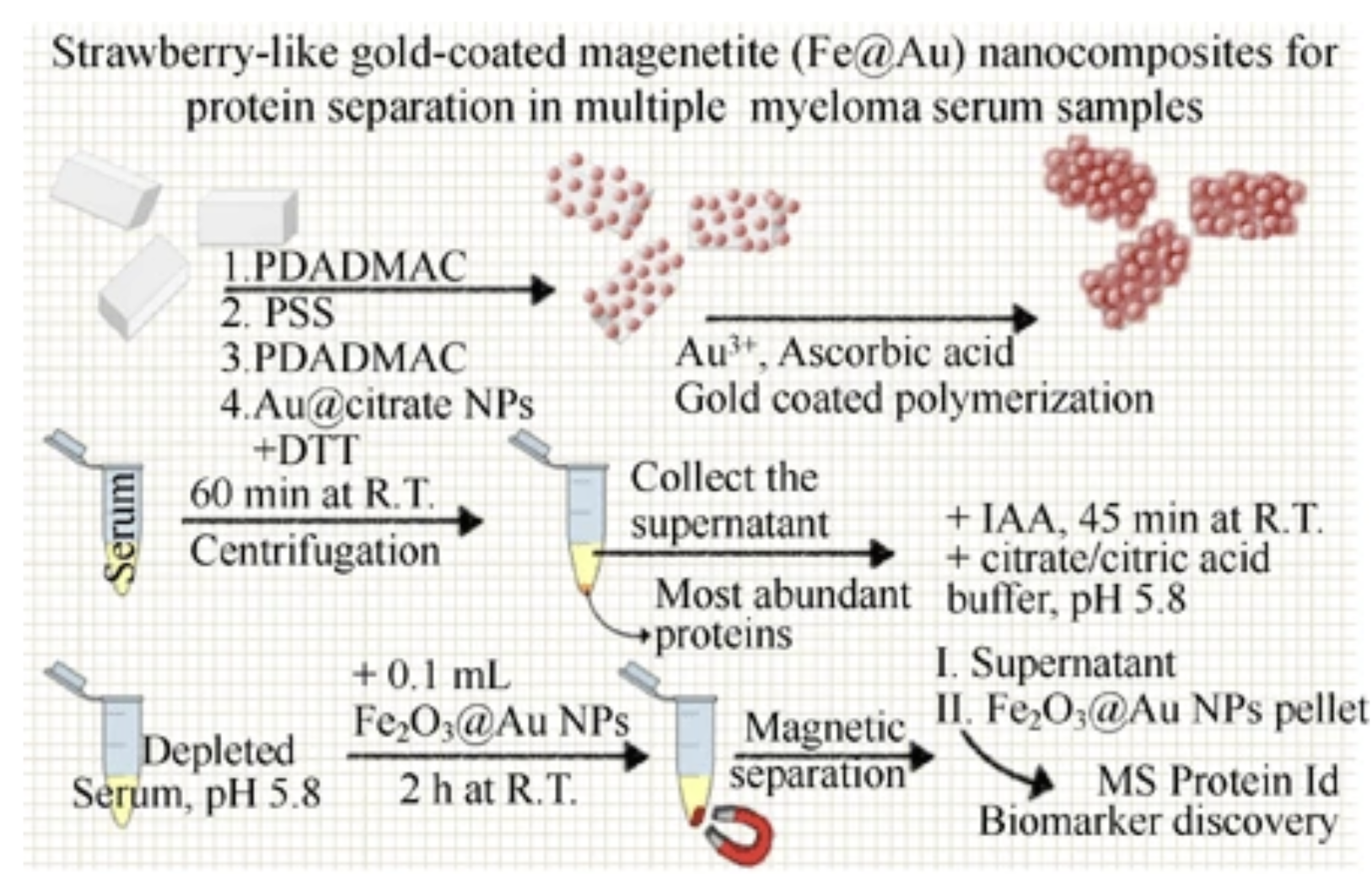
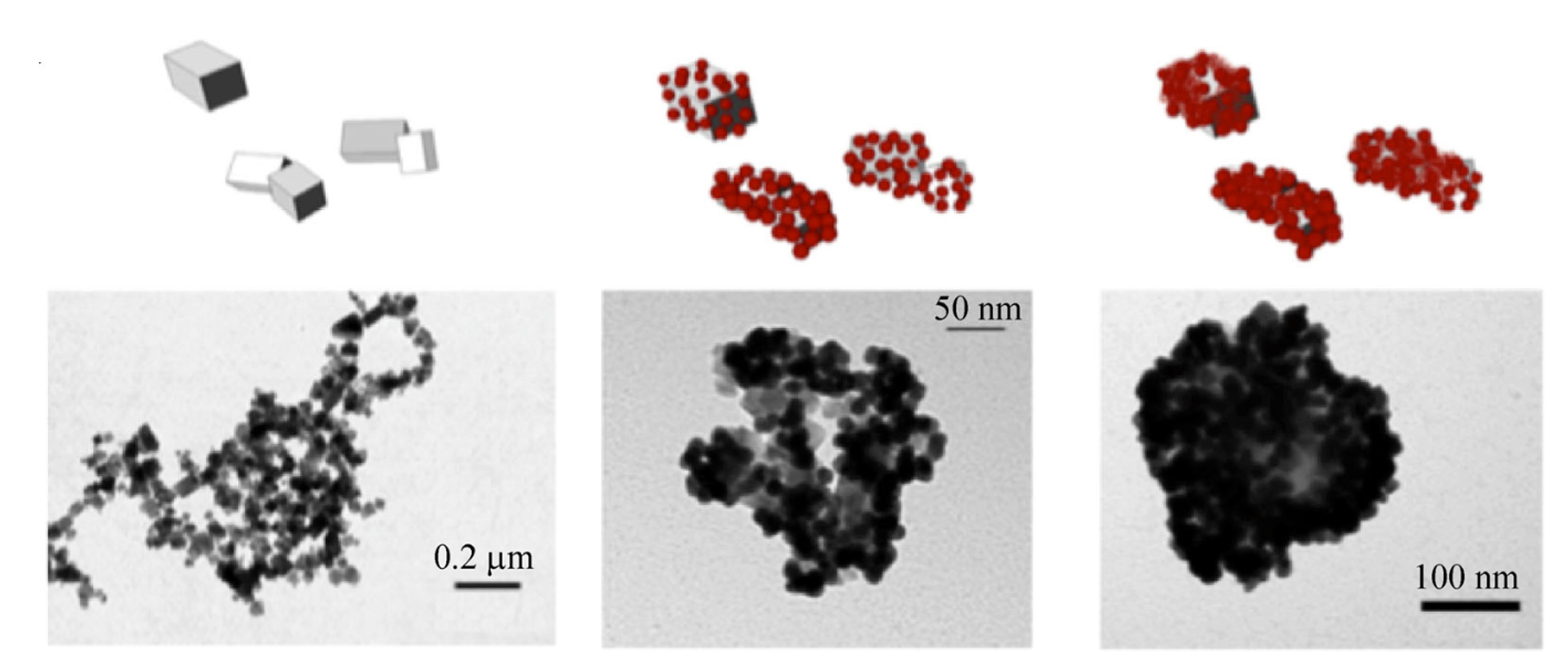
Novel nanocomposites based on a strawberry-like gold- coated magnetite (Fe@Au) for protein separation in multiple myeloma serum samples
A new process to produce magnetite partially coated with strawberry-like gold nanoparticles in aqueous media is reported. The fast response to magnetic fields and optical properties of gold nanoparticle-based colloidal systems are the two main advantages of this new Fe@Au nanomaterial. These advantages allow for the use of this new colloidal nanomaterial for various purposes in proteomics and biomedicine, as proteins can bind to the surface, and the surface can also be functionalized. As proof-of-concept, the new Fe@Au nanoparticles have been assessed in biomarker discovery as a tool for pre-concentration and separation of proteins from complex proteomes. To this end, sera from healthy people were compared with sera from patients diagnosed with multiple myeloma. The application of this new Fe@Au nanomaterial combined with mass spectrometry has allowed for the identification of 53 proteins, and it has also shown that the heat shock protein HSP75 and the plasma protease C1 inhibitor are potential biomarkers for diagnostics and control of multiple myeloma progression. Nano Research, 2015, 8, 1189.
See more at : https://doi.org/10.1007/s12274-014-0599-4
Label-free protein quantification after ultrafast digestion of complex proteomes using ultrasonic energy and immobilized-trypsin magnetic nanoparticles
The ultimate high-throughput, high robustness and easy-to-handling sample treatment method for label-free shotgun proteomics is presented in this work. It is based on joining the effectiveness of immobilized trypsin at the nanoscale level with the latest technology to deliver ultrasonic energy. The new method can be used to reduce sample preparation time comprising the steps of reduction, alkylation and digestion time to just 15 min without compromising shotgun label-free protein quantification. It is demonstrated that trypsin immobilized at the nano-scale performs better than the commercially available counterpart macroparticles. Considering the current advances in (i) ultrasonic energy delivery that allows 96 samples to be treated at once in 30 min, and (ii) chromatography and mass spectrometry for shotgun proteomics, that allow to analyze complex proteomes in 5 min, we envision this methodology as the universal one to digest complex proteomes as it allows to profile quantitatively more than 200 samples per day. Talanta, 2019, 196, 262.
See more at : https://doi.org/10.1016/j.talanta.2018.12.066